
How it works?
An individual and unambiguous identification is assigned to each of the commercialized specialties to be able to track them and ensure the control of the products and contribute to the eradication of the circulation of forged products. It is possible to serialize the primary, secondary or tertiary packaging of the products, according to the regulations of each country or the necessary solution.

Verifarma software allows you to manage serial numbers for pharmaceutical purposes, send and register them by CMO, receive and return the series to MAH (level 4) and manage the series for printing at your own factory. In addition, it allows you to work with third-party software within the plant or to directly manage production lines (level 3, interface availability with 5 different machinery suppliers). At the same time, it is a platform for reporting events online to regulatory entities (EMVO and National Repository, level 5).
Verifarma software gives the pharmaceutical client confidence that he works with a qualified supplier in all necessary fields to comply with traceability regulations. After 10 years of experience, this software has over 2,000 clients using serialization in 10 countries. Knowledge of the industry, details of Directive 2001/83/CE of the European Parliament and delegated act (EU) 2016/161, GS1 coding standards, identification technology, proven computer system, infrastructure with redundancy and security, responsiveness of customer service and preventive service of monitoring reports to the health unit are the key elements that emphasize the service and product which is Verifarma.
Verifarma website
Key Features
- One solution to support process of levels L3-L4-L5, stock, pick&place or parallel import.
- Customer support in regards to legal regulations and requirements of serialization and aggregation
- Process analyse during implementation of the system allows to accommodate the solution perfectly to customer needs
- Cloud service. Access from any device with web browser
- Date protection confirmed by ISO 27001 certificate
- 24/7 customer support with monitoring
- Software updates needed for current regulations changes included in service
- Validation according to GMP
Main benefits
- It reduces or eliminates, the forgery of products
- Eases stock and inventory control
- Improves time for dispatch and reception
- Allows controlling the distribution of the products through the whole commercialization chain
- The customer receives legitimate medication with quality, efficiency, guaranteed and proven safety
- Reduces logistical costs since it detects failures before the remittance of the product

Ask about the product

Hospital & Pharmacy
Verifarma Hospital & Pharmacy is a flexible and complete solution designed specifically for hospitals & pharmacies to be in compliance with the EU FMD requirements.
In order to supply medicinal products in Europe after 9th February 2019 and complete the end-to-end verification, distributors may need to be able to accomplish the verification and decommission of medicinal products at higher risk of falsification in the repositories system.
As expert’s technology providers for the pharmaceutical industry, with Verifarma Wholesalers we help pharmaceutical wholesalers across Europe to cost-effectively ensure compliance with the FMD to minimise the risk of falsified medicinal products.
Functionalities – FMD Transactions:
- Scan 2D data matrix barcode
- Verification of both the authenticity of the unique identifier and the integrity of the anti-tampering device
- ecommissioning of the unique identifier of medicinal products which are:
- Supplied to the public. Supplied status.
- Distributed outside the Union. Export status.
- Intended for destruction. Destroyed status.
- Requested as samples by competent authorities. Sample status.
- Returned products which cannot be returned to saleable stock. Locked status.
- Intends to distribute to the persons or institutions referred to in Article 23*, where required by national legislation in accordance with the same Article.
- Mark as stolen. Stolen status.
- Revert the status of a decommissioned unique identifier to an active status if the conditions described in the article 13 of the Commision Delegated Regulation (EU) 2016/161 are fulfilled.
- Possibility of manual entry the unique identifier code.
- Connection to the National Medicine Verification System of the respective countries.
- Register all events and FMD transactions.
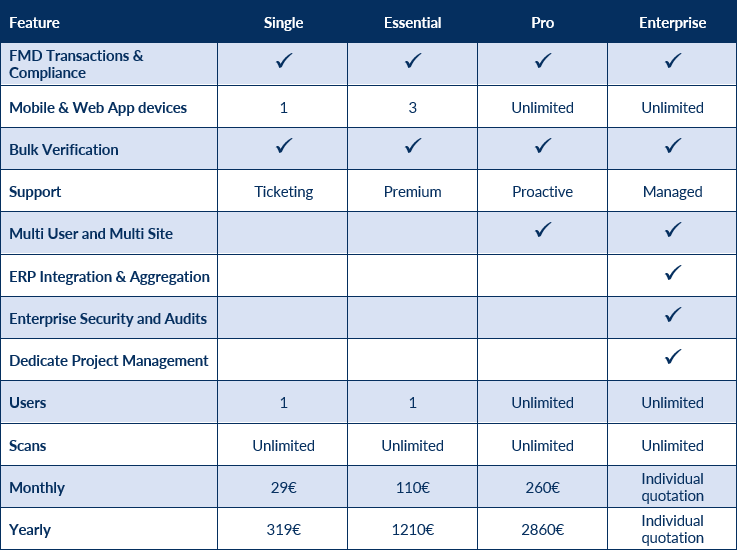
Key Features
- Mobile and web access
- Integrations with client’s ERP
- Rapidly adaptation at regulatory updates
- Cost-effective solution for large, small and medium companies
- Certified quality standards
Wholesalers
Verifarma Wholesalers is a flexible and complete solution designed specifically for pharmaceutical wholesalers to be in compliance with the EU FMD requirements.
In order to supply medicinal products in Europe after 9th February 2019 and complete the end-to-end verification, distributors may need to be able to accomplish the verification and decommission of medicinal products at higher risk of falsification in the repositories system.
As expert’s technology providers for the pharmaceutical industry, with Verifarma Wholesalers we help pharmaceutical wholesalers across Europe to cost-effectively ensure compliance with the FMD to minimise the risk of falsified medicinal products.
When should I verify the authenticity of the unique identifier of a medicine?
- Those returned by other wholesalers or persons authorised or entitled to supply medicinal products.
- Medicinal products distributed by persons who are neither the manufacturer nor a wholesaler holding the marketing authorisation nor a designated wholesaler.
Functionalities – FMD Transactions:
- Scan 2D data matrix barcode
- Verification of both the authenticity of the unique identifier and the integrity of the anti-tampering device
- ecommissioning of the unique identifier of medicinal products which are:
- Supplied to the public. Supplied status.
- Distributed outside the Union. Export status.
- Intended for destruction. Destroyed status.
- Requested as samples by competent authorities. Sample status.
- Returned products which cannot be returned to saleable stock. Locked status.
- Intends to distribute to the persons or institutions referred to in Article 23*, where required by national legislation in accordance with the same Article.
- Mark as stolen. Stolen status.
- Revert the status of a decommissioned unique identifier to an active status if the conditions described in the article 13 of the Commision Delegated Regulation (EU) 2016/161 are fulfilled.
- Possibility of manual entry the unique identifier code.
- Connection to the National Medicine Verification System of the respective countries.
- Register all events and FMD transactions.
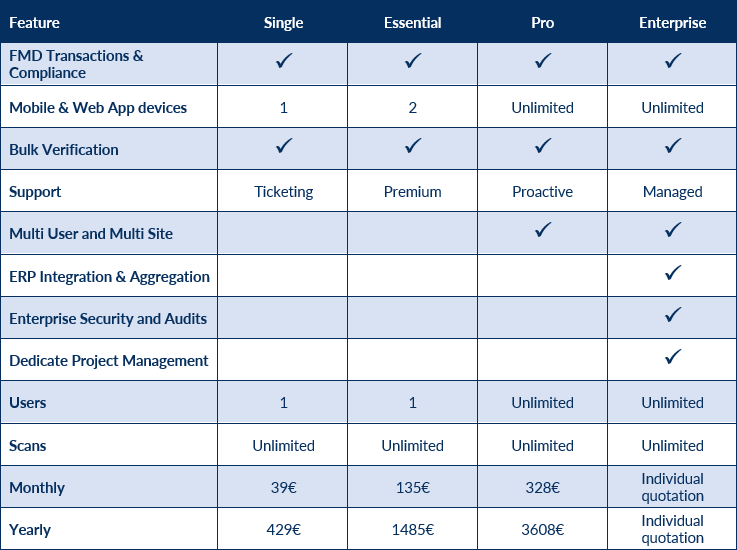

Key Features
- Mobile and web access
- Integrations with client’s ERP
- Rapidly adaptation at regulatory updates
- Cost-effective solution for large, small and medium companies
- Certified quality standards



